Medical Devices that add value to patients and simultaneously capture profitable markets
Successful Medical Device Development
Developing drug-device combinations and e-health solutions – we deliver advanced healthcare solutions that meet patients’ needs.
Therapy improvements by drug-device combination technology and striking e-health solutions mean increased opportunities to meet patients´ needs in challenging global healthcare markets. Our team at TIEFENBACHER MEDICAL DEVICES has extensive knowledge and skills to create innovative solutions – from initial concept ideas through medical device and combination product development and manufacture to post market lifecycle management. We guide and execute along a strategic pathway, focusing on product excellence, quality, and regulatory compliance with patient-centric solutions that enhance the efficacy, safety, and compliance of treatments, for controlled overall therapy costs. Together with our global B2B network, we take those products to global markets.
Founded in 2016 as an innovation hub of the TIEFENBACHER GROUP, TIEFENBACHER MEDICAL DEVICES has rapidly evolved from a medical device spin-off to a cutting-edge multi-disciplinary company for innovative healthcare solutions through digitalization in the pharmaceutical and medical device business. We create value to patients by combing our pharmaceutical expertise with medical device know-how to achieve affordable innovative medical devices and e-health solutions for better therapies.
TIEFENBACHER MEDICAL DEVICES plays an active role in the fast changing and innovative ecosystem of the medical device and pharmaceutical industry. We leverage the full potential by maximizing health outcomes while minimizing healthcare spends.
Experience in every aspect of medical device development
Ensuring compliant Development, Manufacture, and Lifecycle Management for innovative Drug-Device Combination Products
A smart medical device or combination product solution involves the whole customer journey and requires specific interactions in the whole healthcare ecosystem: patient, physician, insurance, healthcare provider, and supplier.
TIEFENBACHER MEDICAL DEVICES helps people to live longer and healthier lives – through passion, innovation, and healthcare expertise. And while companies pursue innovation and safety, many are faced with big regulatory challenges. We orchestrate the business from concept to market and deliver a smart and user-friendly solution to properly satisfy each patient’s demand.
WE ARE BEST PRACTICE
TIEFENBACHER GROUP AND ONDODIS LAUNCH THE INNOVATIVE HEALTHCARE SOLUTION OYSTA®
In 2022 Tiefenbacher Group and Ondosis launched the new healthcare brand OYSTA®. With OYSTA® we want to revolutionize how patients take their medicines so that every individual precisely gets the right dose of the medicine they need in a simpler, more comfortable way. We combine pharmaceuticals and technology to guarantee intuitive, precise, and flexible dosing via a connected handheld device and follow up in digital solution.
OYSTA® aims to improve the health of patients with various diseases, such as ADHD, rare pulmonary diseases, and transplantation medicine through individualized dosing, safe medication management, and treatment support. By enabling optimized dosing of medication based on solid patient reported data, OYSTA® is also intended to make it easier for healthcare providers to improve adherence, adjust treatment, and monitor progress.
For this purpose, we offer two stand-alone solutions that provide synergistic clinical value when paired: the OYSTA® Dosage Manager (digital dispensing device, including medicine) and OYSTA® Digital Service. OYSTA® Dosage Manager and Digital Service offer a comprehensive solution for patients, caregivers, and providers seeking a holistic and optimal treatment.
Please visit oysta-health.com to learn more about our innovation in the field of precision medicine!
*Dosage Manager and Digital Service are currently under development and have not been cleared or approved by the U.S. Food and Drug Administration.

Dr. Mathias Mönckedieck
TIEFENBACHER MEDICAL DEVICES: Project Management
„We generate value for patients through innovative smart medical device and combination product solutions. Our way to hit the target: with passion for innovation, longstanding expertise in medical device engineering and entrepreneurial spirit in a dynamic team."
„We stay ahead of the curve in this dynamic healthcare business by embracing an innovative approach of more patient-centered design. We leverage our expertise in pharma, medical devices, and digital healthcare to create better therapeutic solutions for many patients worldwide."

Dr. Marie Inken Wiechert
TIEFENBACHER MEDICAL DEVICES: Business Development & Market Entry
Dedication to innovation, technology, and continuous improvement
Participate in future innovations with TIEFENBACHER MEDICAL DEVICES
We bring new medical device and e-health concepts to market in the shortest possible time and cost-competitively.
TIEFENBACHER MEDICAL DEVICES converts drug-delivery device concepts into market-ready innovative products connectable with optional eHealth applications. During the concept phase we understand all technical and functional needs together with regulatory requirements of the prospective medical device for EU + US markets. Controlling the conversion of concepts to commercial manufacturing, supply chains as well as verification and validation of the device design are the key elements of our development strategy. For marketed products, we cover and maintain all lifecycle activities such as device vigilance, post-market clinical follow-up, post-market surveillance, risk management, clinical evaluation and quality audits. Our smart solution improves the quality and outcome of a treatment by simplifying processes and saving time and money – with continuous iterative-incremental product improvement over time.
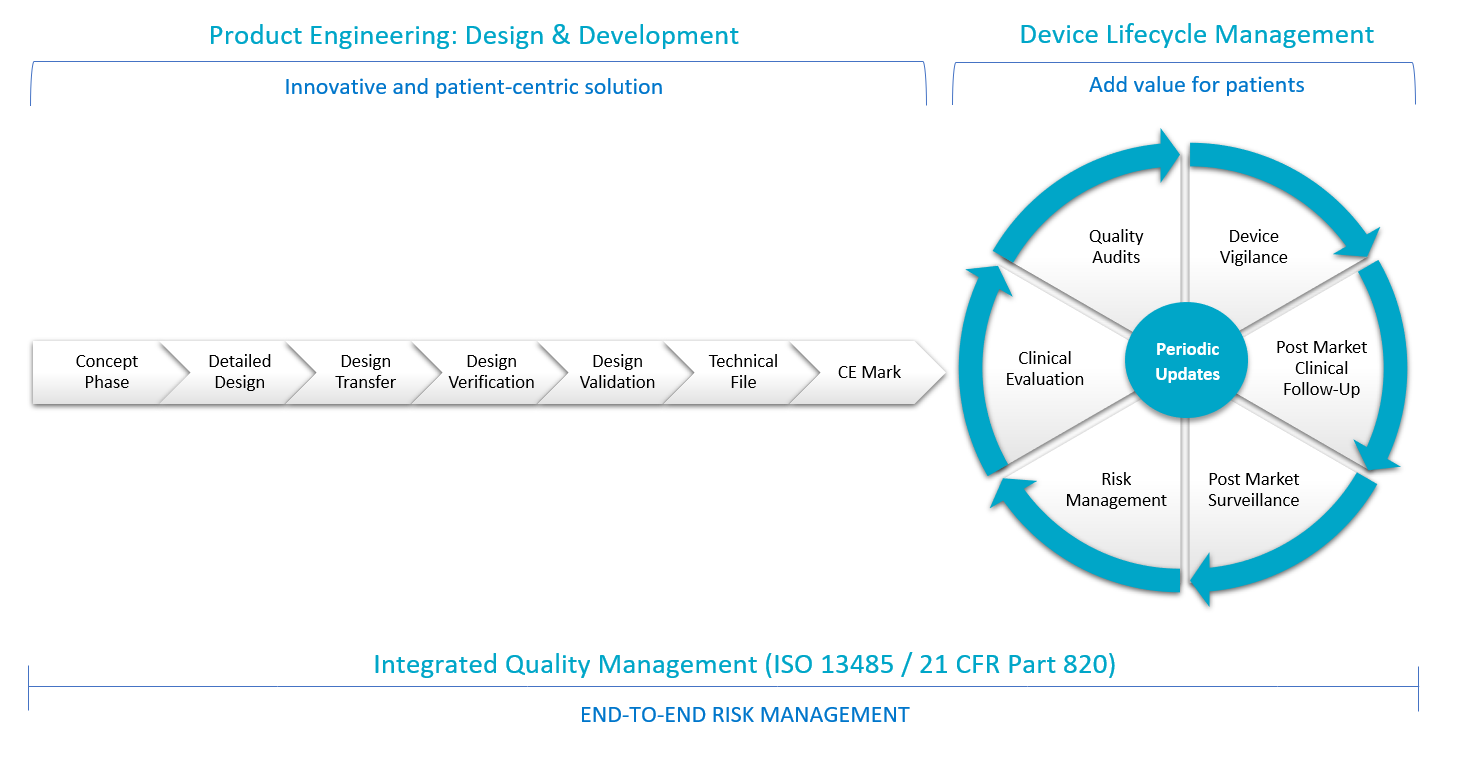
Our multidisciplinary technological expertise contributes to an innovative medical device and digital transformation process – while responding to growing demands for cost-saving solutions in the healthcare industry
Our innovation unit has experience in every aspect of medical device development including digital solutions. Specialists for design, usability, risks, engineering, injection molding, assembly, metrology, quality, regulatory, medical, legal, and business - all work together towards a common goal – adding value to therapies and patients´s lives. Partner with us to to participate in success for future projects. We offer tailor-made healthcare innovations ensuring efficiency and quality to establish a strong position in the market for future growth.
The commercial success of a product is critical in maximizing its potential and the shareholder return. Contact us for innovative medical devices and eHealth solutions – or would you like to discuss your upcoming innovation project with us, please reach out to our team by filling out the contact form below and clicking “Send message”, or please directly send an email to info@tiefenbacher.com. We, at TIEFENBACHER, will follow up with you personally as soon as possible.


